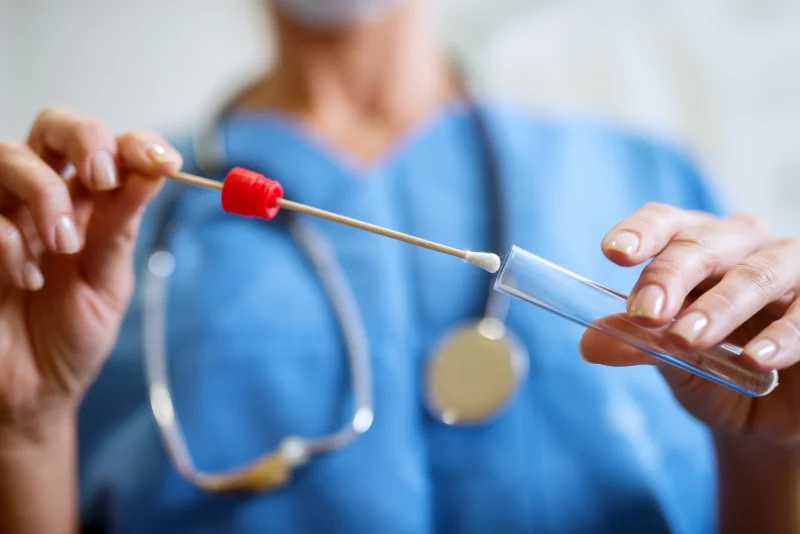
The simple act of providing a saliva sample could soon play a much larger role in modern medicine. Researchers and clinicians are exploring how the biological information contained in a few drops of spit might help detect diseases earlier, monitor chronic conditions and reduce barriers to care. Because saliva collection is noninvasive and painless compared to blood draws, it holds particular appeal for routine screenings in both medical and dental settings.
Today, saliva-based diagnostics are already used to detect certain viral infections, including HIV and COVID-19. Genetic saliva tests are also available to evaluate inherited risks for diseases such as breast cancer. Scientists believe the next wave of development could expand this technology to identify conditions ranging from diabetes to prostate cancer, potentially shifting health care toward earlier and more preventive intervention.
Expanding Clinical Potential and Regulatory Limits
Saliva contains a complex mix of enzymes, hormones, antibodies and microbial signatures that reflect processes occurring throughout the body. That biological richness has made it a promising diagnostic medium. Organizations such as the American Dental Association have emphasized that salivary biomarkers could one day complement traditional medical screenings performed during routine dental visits.
However, widespread adoption remains limited. While certain laboratory-developed tests are permitted under federal oversight, only a narrow range of saliva diagnostics has received full clearance from the Food and Drug Administration (FDA). FDA approval requires extensive clinical validation to ensure consistent reliability and accuracy across diverse populations.
Many companies currently operate under regulatory pathways that allow testing through specific certified laboratories rather than broad FDA authorization. These tests, which often cost between $100 and $200, are typically purchased online or administered in dental offices, with samples mailed to centralized labs for analysis. Without insurance reimbursement, cost remains a significant barrier for many patients.
Insurance Incentives and the Push for Approval
A new policy shift may accelerate innovation. Under recent federal spending legislation, Medicare is required to cover FDA-approved multi-cancer detection tests, regardless of whether they rely on blood, saliva or other biological samples. The Centers for Medicare & Medicaid Services (CMS) will play a key role in implementing this coverage once qualifying diagnostics receive approval.
That financial incentive has prompted several biotechnology firms to pursue full FDA clearance for saliva-based cancer screening tools. If successful, the change could open national reimbursement channels and encourage private insurers to follow Medicare’s lead. Broader coverage would make such tests more accessible and potentially integrate them into preventive care strategies.
Companies developing these diagnostics argue that early detection could reduce long-term treatment costs and improve survival rates. For conditions like head and neck cancers, where symptoms may be subtle in early stages, reliable saliva tests could help identify disease before it advances.
Scientific Challenges and Future Applications
Despite its promise, saliva testing presents technical complexities. Unlike blood, saliva composition fluctuates throughout the day. Eating, drinking, brushing teeth and smoking can alter its chemical and microbial balance. Researchers supported by institutions such as the Centers for Disease Control and Prevention are working to isolate consistent biomarkers that remain stable despite these variations.
Scientists describe saliva as a kind of microbial fingerprint unique to each individual. That variability complicates the search for universal disease markers but also offers opportunities for personalized diagnostics. Advances in molecular analysis and machine learning may help identify patterns that reliably correlate with specific illnesses across large populations.
In clinical practice, saliva tests are already being used experimentally to monitor post-surgical infections and support telehealth care, particularly for patients in rural areas or those with limited mobility. As research progresses and regulatory pathways evolve, saliva-based disease detection may become a routine component of preventive health, complementing — rather than replacing — traditional in-person medical and dental evaluations.