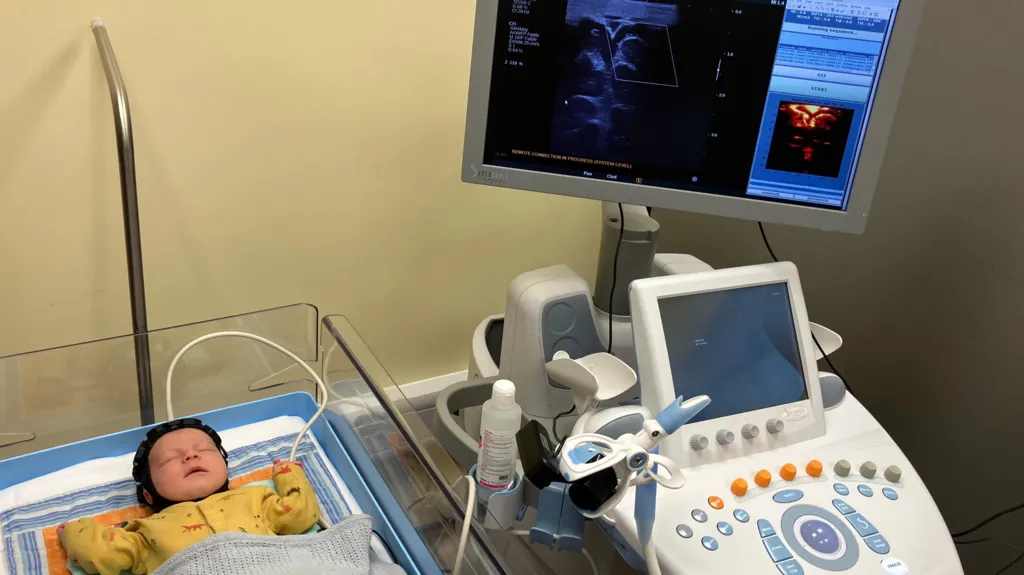
A Breakthrough in Neonatal Brain Monitoring Technology
At just three weeks old, baby Theo became part of a groundbreaking trial at the Cambridge University Hospitals NHS Foundation Trust. A small, cap-like device with hexagonal sensor modules was placed on his head. This innovative device uses both high-density diffuse optical tomography (HD-DOT) and functional ultrasound (fUS) to monitor brain activity in newborns at risk of neurological injury.
According to the disclosed project details, the dual-modality headset is designed to monitor whole-brain functional connectivity at the cot-side. This approach is preferred rather than relying solely on traditional methods like magnetic resonance imaging (MRI) or cranial ultrasound (CUS). cordis.europa.eu+2cordis.europa.eu+2
The wearable cap is described as “portable.” It enables repeated scans in the neonatal unit without moving the infant to a scanner bed.
The light sensors monitor changes in oxygenation around the brain surface. Meanwhile, the ultrasound sensors assess micro-blood vessel structure and activity deep inside the brain. Supporters of the innovation suggest that if deployed widely, the technology could transform early detection and intervention for conditions such as cerebral palsy, epilepsy, and other developmental disorders.
Why This Technology Matters for Early Intervention
Premature birth and neonatal brain injury remain leading causes of lifelong disability. Current imaging approaches have limitations. MRI is costly, requires transporting the infant, and often cannot be repeated frequently. Meanwhile, cranial ultrasound may miss subtler lesions or deeper brain connections. The new approach is significant because it combines two advanced modalities: HD-DOT and fUS. These deliver a more complete functional picture of the neonatal brain. PubMed+1
Researchers cite that by capturing functional connectivity patterns in the first days and weeks of life, it becomes possible to identify which infants are at highest risk of later developmental problems. Early identification means therapies, monitoring, and parental counselling can begin earlier. This potentially improves outcomes and reduces long-term care costs. The device may also reduce the reliance on multiple expensive scans. Additionally, it might prevent delayed diagnoses, thereby offering a more equitable model of neonatal care.
Implementation and Future Outlook for Widespread Use
The clinical team at Cambridge has indicated that the device could be available in UK hospitals within three to five years, assuming successful trials and regulatory approval. The project is part of a larger study known as FUSION (Functional UltraSound integrated with Optical Imaging in Neonates). It is funded in part by the European Union under the Horizon programme and coordinated by the University of Cambridge.
cordis.europa.eu+1 Implementation will require device certification and training of neonatal staff. It also needs integration into existing neonatal care pathways, and expansion of therapy capacity for infants identified as high risk. Charities such as Action Cerebral Palsy have welcomed the innovation. However, they caution that community therapists must be ready to meet increased demand for early intervention services. If successful, the cap may transition from being a specialist tool to a standard screening device in neonatal units. This would enable cost-effective and routine monitoring of infants at risk for brain injury.